Mechanical Force and Leukocyte Adhesion
When a tissue becomes injured or infected, the endothelial cells that line the blood vessels receive distress signals from the tissue that cause them to increase their expression of adhesion molecules and signaling molecules on their surface. Complementary molecules on white blood cells circulating in the blood (in our case, neutrophils) bind to these endothelial cell ligands, causing the neutrophils to roll along the vessel wall, stop, and then crawl through the endothelium into the tissue to fight the infection. As a neutrophil is rolling along the endothelium, attachments at the rear of the cell in combination with fluid forces pushing the cell downstream combine to create compression between the cell and the substrate toward its leading edge. Understanding how these forces may modulate the adhesive interaction is important for being able to predict cell behavior under different conditions.
In our lab we have determined the effects of force on adhesion by using micropipettes to control to force of interaction between cells and bead surfaces coated with adhesion molecules from endothelial cells. We find that the magnitude of the contact stress (force per unit area) of the contact zone and the area of the contact zone each produce a proportional increase in cell adhesion over a range of conditions.
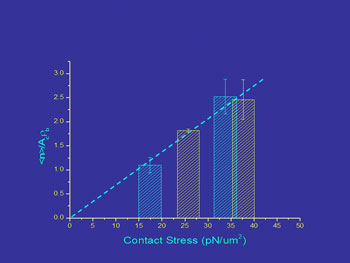
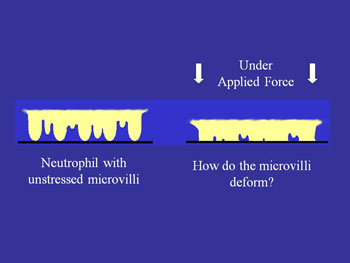
Left: Contact stress effects on bond formation. Right: Unstressed microvilli v. microvilli when force is applied.
A second way in which mechanical force may affect adhesion arises from the fact that cell (leukocyte) surfaces are not smooth, but consist of numerous microvilli or ruffles. Changes in the surface topography as a cell is compressed against a substrate may have a strong effect on adhesion because adhesion molecules are not uniformly distributed on the cell surface. Rather, some (like L-selectin) are concentrated near the tips of microvilli while others (like integrins) tend to be found in the valleys between the microvilli (see schematic). Thus as a cell gets pressed against a substrate the concentration of molecules close enough to the surface to from a bond should increase as the irregularities on the cell surface are compressed.
 Left: Donor 1.
Left: Donor 1.
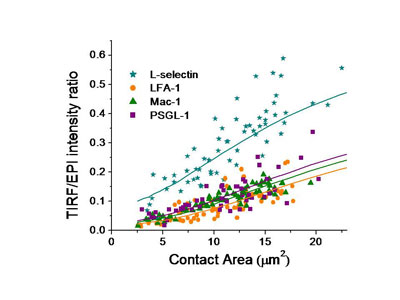
To understand the importance of this in neutrophil adhesion, we developed a mechanical model of the interface and generated predictions for what percentage of the molecules on the convoluted cell membrane would fall within a certain distance of the surface. We then compared that with measurements of fluorescence intensity in total internal reflectance fluorescence microscopy (TIRFM), which illuminates only those molecules within 200 nm of the surface. The ratio of the TIRFM intensity to the fluorescence intensity in ordinary fluorescence illumination is predicted to be different depending on whether molecules are distributed near the tips of microvilli on the cell surface or in the valleys in between the microvilli.
We tested four of the main adhesion molecules on the cell, and confirmed that L-selectin is located at microvilli tips, and that the beta-2 integrins LFA-1 and Mac-1 are distributed away from the tips, consistent with what had been seen in fixed preparations in an electron microscope. Interestingly, we found that PSGL-1, a molecule that mediates cell rolling, was not located at microvilli tips, contradicting an earlier report and correcting an important error in the literature.
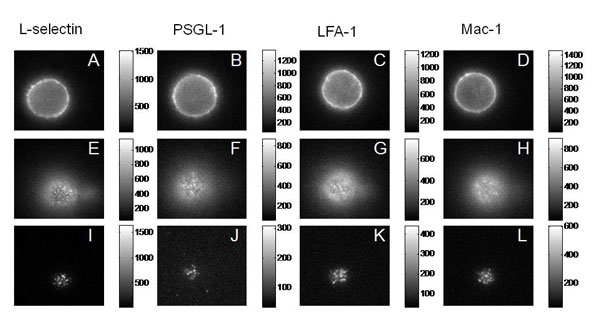
Fluorescence (Epi and TRIF) Images of the Major Adhesion Molecules
Researchers:
Richard E. Waugh, Ph.D.
Cell Adhesion, Mechanical and thermodynamic properties of biological membranes; cellular mechanics and function of cytoskeletal proteins
Elena Lomakina, Ph.D.
Cell Adhesion, Mechanical and thermodynamic properties of biological membranes; cellular mechanics and function of cytoskeletal proteins
